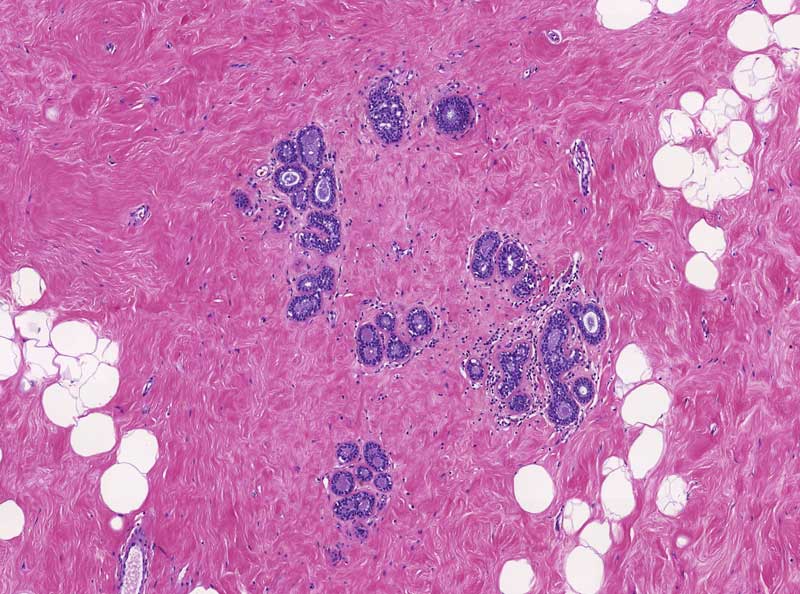
Microscopic details of healthy mammary cells.
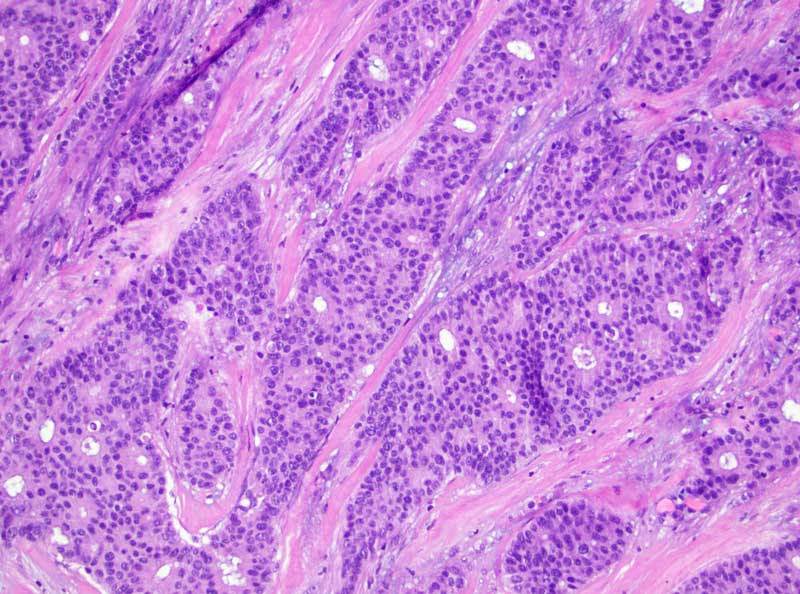
Microscopic details of cancerous cells.
Research
At the IBCC, we lead multiple national and international studies and clinical trials aimed at improving the diagnosis and treatment of breast cancer. From our Clinical Research Unit and Clinical Trials, we work intensively to achieve the following objectives:
1. Conduct clinical studies on breast cancer patients in all stages of development (I-IV), establishing the unit as a national and international reference center for clinical trial development.
2. Facilitate access to innovative drugs with significant therapeutic potential for our breast cancer patients.
3. Provide breast cancer patients with the opportunity to receive treatments not covered by their insurance, avoiding referrals to Public Health Hospitals.
4. Promote the establishment of a biological registry (biobank and serum bank) with the aim of participating in national and international translational research projects focused on breast cancer.
5. Increase the acquisition of breast cancer research projects and facilitate the transfer of clinical research project results into clinical practice, promoting innovation and healthcare quality.
6. Boost the publication of articles in high-impact scientific journals to increase the visibility of the center.
